* Diffusion is of the Solute
* Osmosis is of the water
1. Problems using diffusion and osmosis
The solutions in the two arms of the U-tube are separated by a membrane that is permeable to water and glucose but not sucrose. Side A is half filled with a solution of 2M sucrose and 1M glucose. Side B is half-filled with 1M sucrose and 2M glucose. Initially, the liquid levels on both sides are equal.
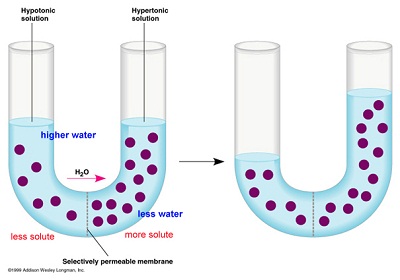 Initially in terms of tonicity, the solution in Side A with regards to that is Side B is isotonic. (3M for each).
Initially in terms of tonicity, the solution in Side A with regards to that is Side B is isotonic. (3M for each).After the system reaches equilibrium, what changes are observed?
Diffusion .5M glucose B-->A osmosis water B-->AThe solutions in the arms of the U-tube are separated at the bottom of the tube by a selectively permeable membrane. The membrane is permeable to sodium chloride but not to glucose. Side A is filled with a solution of 0.4 glucose and 0.5M of NaCl, and Side B is filled with a solution of 0.8M glucose and 0.4M NaCl. Initially, the volume in both arms is the same.
At the beginning of the experiment Side A is hypo to Side B. Side A after 3 days, 0.5 NaCl B --> A and Water A-->B
2. Notes from the reading (because of the shortened schedule we did not have the chance to cover all of Sec. 7.3 and will finish on Tuesday)
Ions have both a chemical and a voltage gradient across the membrane, and this causes an ELECTRICHEMIC GRADIENT. The inside of the cell is slightly more negative than the outside, so that membrane potential favors the movement of citations (positively charged ions) into the cell
The sodium potassium pump works by exchanging sodium Na+ for potassium K+ across the cell membrane; it exchanges 3 Na+ ions for 2 K+ ions, so for each round of the pump, there is a net transfer of one positive from the cell interior to the exterior. This type of pump, called ELECTROGENIC PUMP, generates voltage across the membrane.

<-- Osmotic Gradient

Vocabulary to review from this section:
Diffusion: the movement of molecules of any substance so that they spread out evenly into the available space
Concentration Gradient: the region along whicht the density of a chemical substance decreases
Passive Transport: the diffusion of a substance across a biological membrane
Osmosis: the diffusion of water across a selectively permeable membrane
Tonicity: the ability of a solution to cause a cell to gain or lose water
Isotonic: having the same solute concentration as another solution, thus having no effect on passage of water into or out of the cell.
Hypertonic: comparing two solutions, referring to the one with greater solute concentration Hypotonic: comparint two solutions, referring to the one with less solute concentration
That's all ladies! Have a great weekend! Get some rest, go to your conferences, study for our quiz on Monday, tell your parents you love them, and above all keep yourselves very safe!!!
2 comments:
GREAT JOB MEGAN!
Great example of the molarity questions. I think that I will add questions and problems to your post as well. This will be a good explanation for those absent.
Post a Comment